The solution came from National Instruments and combined LabVIEW software with a broad range of NI Hardware, including PXIe, CompactRIO, myRIO and Diagnostic Sonar’s FlexRIO-based FIToolbox, to implement a full-featured system taking the Sonopill prototypes from initial characterisation through encapsulation to final deployment for pre-clinical validation.
Sonopill encompasses microelectronic sensors, including ultrasonic technology, to perform advanced health diagnostics as it travels through a patient’s gastrointestinal tract. It is the outcome of a five-year, $10m programme, established to develop a multimodal capsule endoscopy device, including ultrasound and other capabilities. It involves four university partners in the UK, Glasgow, Dundee, Heriot-Watt and Leeds, and features a multi-disciplinary team of researchers ranging from electrical and mechanical engineers to life scientists and clinical fellows – all of whom have used NI hardware and software.
In 2010, there were almost 50 million visits to doctors in the US for gut related disorders. In the UK, 20-40% of the population report gastric conditions. Clearly, there is an urgent need for practical and accurate diagnosis and treatment options.
Current clinical endoscopy uses conventional devices, which are inserted into an orifice and manually controlled via external manipulation, with limited reach. These devices rely mainly on high definition optical sensors, with some also supporting low to mid-frequency ultrasound sensors. The last three decades have also seen the development of capsule endoscopy devices capable of passing through the entire gastrointestinal system. However, these systems are limited to lower definition optical imaging and do not exploit ultrasound imaging at all.
Miniaturised sensors
To integrate ultrasound imaging into a capsule-sized device requires the highly miniaturised sensors and electronics, capable of operating in a power-limited, hermetically-sealed enclosure measuring 20-30mm (length) and 10mm (diameter). There are currently no commercial components that can meet these demanding system specifications – so sub-components of the Sonopill capsule had be developed from scratch and tested on the bench.
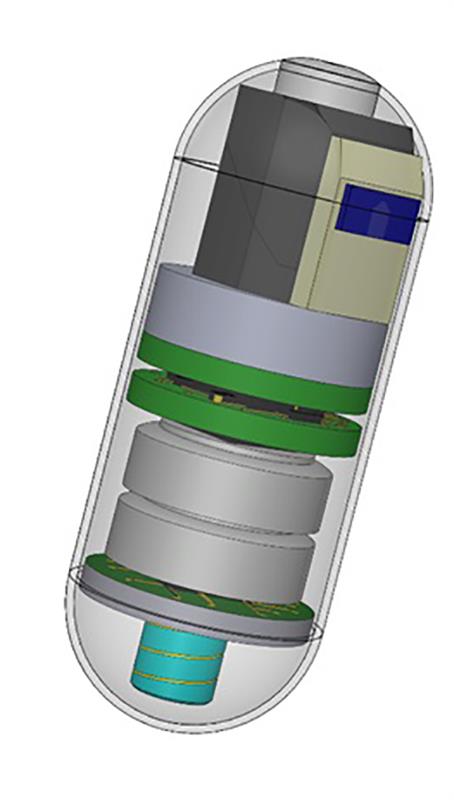 Once developed, these sub-components must then be functionally tested in isolation, then retested during system integration into the final capsule. These tests include measuring of basic device parameters, such as signal integrity and power usage, as well as the replication of imaging modalities for benchmarking image quality and sensing capabilities.
Once developed, these sub-components must then be functionally tested in isolation, then retested during system integration into the final capsule. These tests include measuring of basic device parameters, such as signal integrity and power usage, as well as the replication of imaging modalities for benchmarking image quality and sensing capabilities.
Finally, all capsules must be tested pre-clinically in vivo to establish safe operating conditions. These tests are done in a specialised facility with a variety of prototype devices, requiring a robust instrumentation solution, with a wide range of data logging and control options.
One of the primary goals was to identify a single-vendor instrumentation solution that allowed replication of tests and results across all sites without significant equipment transport.
NI emerged as providing the best combination of equipment capability, flexibility, customisation and customer service. LabVIEW provides an intuitive means of building complex systems and allows the seamless integration of NI hardware along with specialised equipment, such as ultrasonic pulser/receivers and high-resolution motor controllers, using third-party LabVIEW drivers. These capabilities made NI products the winning solution.
To maximise the usability of the systems during and after development, NI provided instructor-led training for team members, as well as on-going support through its field sales engineers, who helped with developing the specifications of the required equipment for all phases. The online support, from both NI staff and the wider LabVIEW community, was also pivotal in the development and debugging of our various test software applications.
The NI platform provided a unifying system development experience, allowing us to transfer code assets seamlessly between sections of the project and efficiently handover entire systems when our engineers moved on to other tasks.
When establishing the testing requirements, the team identified several discrete phases in the device development, with the corresponding instrumentation solutions detailed below.
Ultrasound capsules use the natural motion of the human gastrointestinal tract to allow linear scanning of the full length of tissue. As this motion was not present during preliminary tests, a motorised scanning and integrated ultrasound generation and data capture system was developed. The team acquired a FlexRIO PXIe-1071 chassis, housing a PXIe-8360, NI-5772, PXIe-7966 and PXIe-5451, and connected it to a pair of linear motors and an ultrasonic pulser/receiver.
A PXIe-7966 FlexRIO module, coupled with a NI-5772 digitiser adapter module, to acquire data sets at very high repetition rates and to synchronise the pulser/receiver.
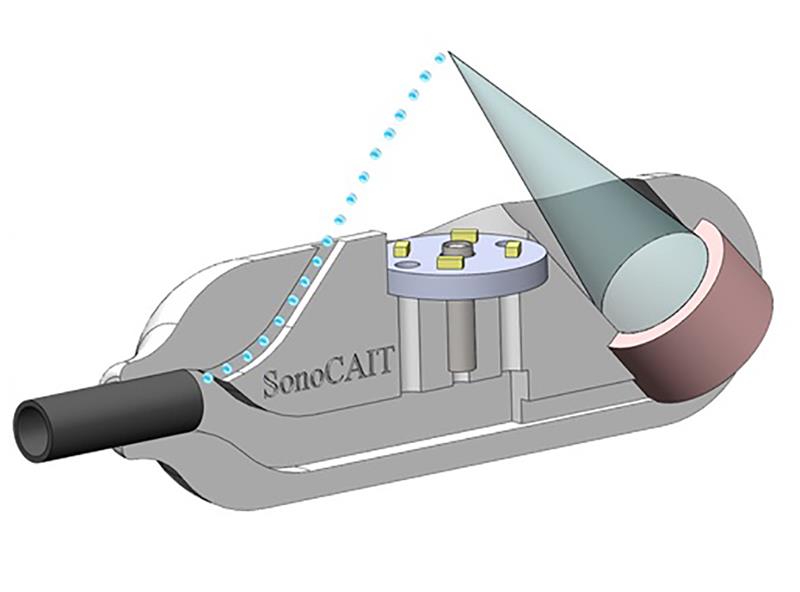 To ensure intuitive control of the motors, custom LabVIEW-based GUIs that were suitable for use by research students and clinical users were written. The team captured the data with high-speed FIFO buffers on the PXIe-7966’s integrated FPGA, before streaming the data to the desktop application using a 100MB/s MXI link (established through a PXIe-8360).
To ensure intuitive control of the motors, custom LabVIEW-based GUIs that were suitable for use by research students and clinical users were written. The team captured the data with high-speed FIFO buffers on the PXIe-7966’s integrated FPGA, before streaming the data to the desktop application using a 100MB/s MXI link (established through a PXIe-8360).
For array devices, an FI was used to supply electronic focusing and steering of the ultrasonic beams. Its FlexRIO-based integrated solution allowed full control of the formation of the ultrasound beam during transmission and reception, along with full data capture - a critical requirement for device performance analysis and benchmarking.
For testing sensors for pressure and pH, a multi-functional test platform comprising a CompactRIO (9035) with a wide range of IO modules (such as NI 9220, NI 9485, NI 9237, NI 9505, NI 9403, NI 9264 and NI 9214) was acquired.
As the work took place on-site at the University of Edinburgh’s Dryden Farm facility, the team required an equipment controller/data logger solution that would adapt well to various test conditions and sensors while remaining compact and highly portable. It identified the myRIO-1900 as the best solution for its variety of IO ports, small footprint, and intuitive programming experience. This last point was key to ensuring students could use myRIO to quickly develop their sensor testing systems, without undue impact on their thesis progress.
To date, the team has used the myRIO in the testing of prototype devices featuring wireless data antennas (RFCap), temperature and power measurement circuits (ThermoCap) and high-frequency ultrasound sensors (SonoCap).
The development of this instrumentation has also allowed the collection of data critical for four PhD theses, the contents of four book chapters, and 22 peer reviewed journal papers (including IEEE).
Early results from the Sonopill prototypes, developed using the NI based test-bed, have directly led to the submission of a follow-up MultiPill grant proposal (NI-supported) worth a total of $10m. If funded, it will take the research from preclinical measurements to the first-in-human testing. This is a critical and necessary step to bringing the technology into clinical use – where it has the potential to fundamentally impact health outcomes for many millions of people suffering from gastrointestinal diseases.
The MultiPill grant will focus on the challenges of integration necessary for first-in-human trials and multimodal functionality. The level of inter-site coordination and communication that will be required to achieve this will demand standardisation of the existing solutions across all relevant work sites, with a particular focus on developing a portable instrumentation, based on a current myRIO-based in vivo test rig, which can be deployed on short notice to any site in the UK.
The Sonopill programme also launched investigation of the use of robotic manipulation for positioning and localisation of capsules during passage through the human body. MultiPill will place a much stronger emphasis on this aspect of the work and it is expected that future instrumentation developments, that will include NI instrumentation, will feature direct feedback between the ultrasonic, diverse electronic sensors and robotic control systems.











