The surprising and counterintuitive finding could have serious implications for the design of certain coating processes or for 3D printing, which both require getting materials to stick together and stay that way.
The research, carried out by MIT researchers Mostafa Hassani-Gangaraj and David Veysset and professors Keith Nelson and Christopher Schuh, outlines “a revolutionary advance in the technology” for observing extremely high-speed interactions, and makes use of that to reveal that melting induced by impacting particles of metal can impede bonding.
The optical setup, with a high-speed camera that uses 16 separate charged-coupled device imaging chips was primarily developed by Veysset. The camera is so fast that it can track individual particles being sprayed onto a surface at supersonic velocities, a feat that was previously not possible. The team used this camera, which can shoot up to 300 million frames per second, to observe a spray-painting-like process similar to ones used to apply a metallic coating to surfaces in many industries.
“You can’t see it, you can’t tell what’s happening, and no one has ever been able to watch the moment when a particle impacts and sticks,” Prof Schuh says. “As a result, there has been ongoing controversy about whether the metal particles actually melt as they strike the surface to be coated. The new technology means that now we can watch what’s happening, can study it, and can do science.”
The images make it clear that under some conditions, the particles of metal being sprayed at a surface really do melt the surface — and that, unexpectedly, prevents them from sticking. The researchers found that the particles bounce away in much less time than it takes for the surface to resolidify, so they leave the surface that is still molten.
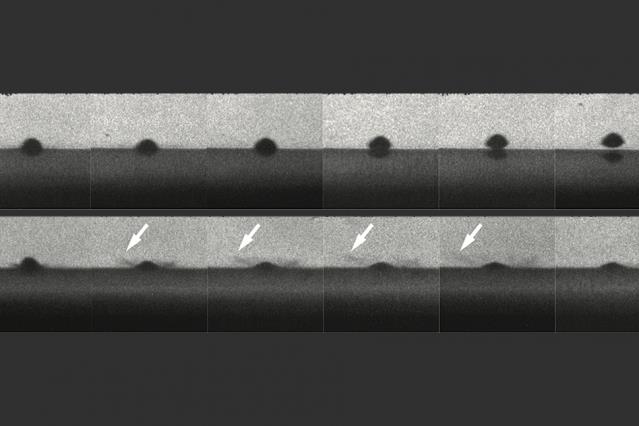 The top row of photos shows a particle that melts the surface on impact and bounces away without sticking. The bottom row shows a similar particle that does not melt and does stick to the surface. Arrows show impact sprays that look like liquid, but are actually solid particles.
The top row of photos shows a particle that melts the surface on impact and bounces away without sticking. The bottom row shows a similar particle that does not melt and does stick to the surface. Arrows show impact sprays that look like liquid, but are actually solid particles.
If engineers find that a coating material isn’t bonding well, they may be inclined to increase the spray velocity or temperature in order to increase the chances of melting. However, these results show that melting should be avoided.
According to Prof Schuh, the best bonding happens when the impacting particles and impacted surfaces remain in a solid state but “splash” outward in a way that looks like liquid.
“That phenomenon is found in a variety of these metal-processing methods,” he said. “To stick metal to metal, we need to make a splash without liquid. A solid splash sticks, and a liquid one doesn’t.”
Now, with the ability to observe the process by precise measurements, the researchers have found the conditions needed to induce that bond.
The findings could be relevant for processes used to coat engine components in order to reuse worn parts rather than scrapping them.
“With an old engine from a large earth-moving machine, it costs a fortune to throw it away, and it costs a fortune to melt and recast it,” Prof Schuh explained. “Instead, you can clean it off and use a spray process to renew the surface.”





